Bohr's atomic model — overview & importance Level bohr energy diagrams chapter ppt powerpoint presentation 4e2 bohr model
Bohr Model of the Atom - Overview and Examples
Bohr atom model explained examples
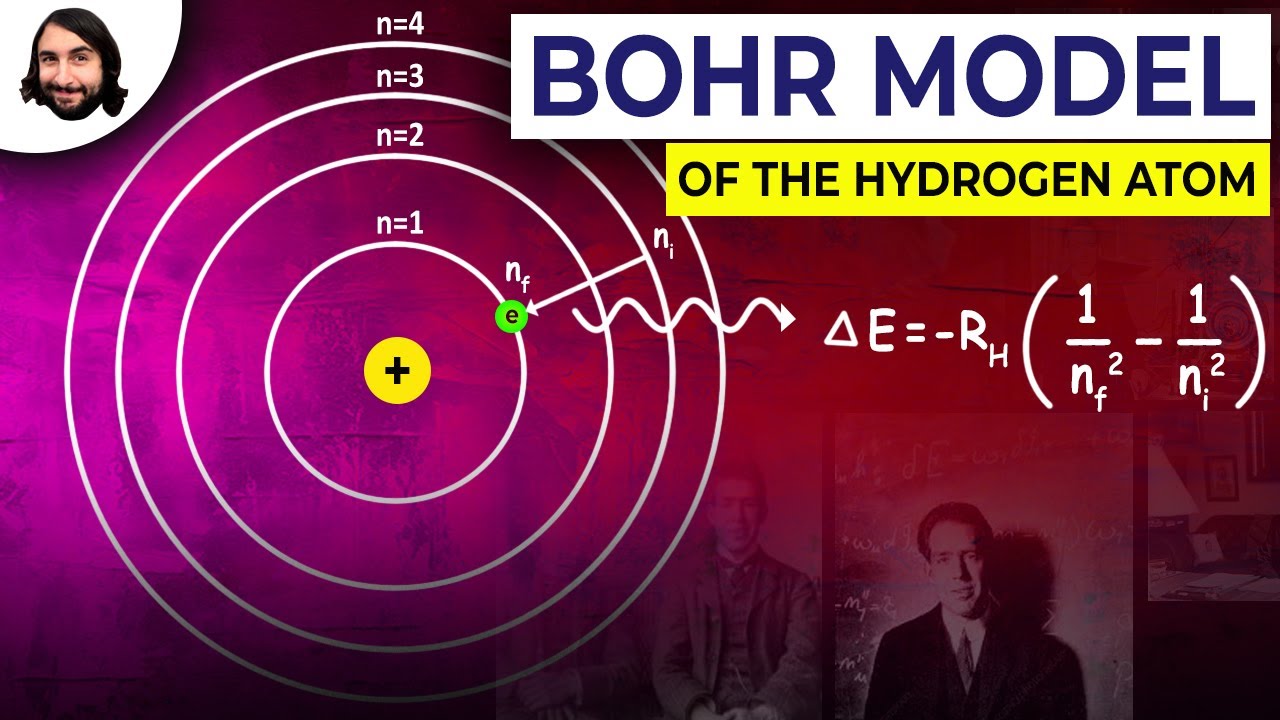
Bohr’s theory of the hydrogen atom
The bohr model can be used to describe which speciesBohr model of atom Atom model energy chemistry bohr hydrogen diagram levels emission electron relative figure electrons quantum lines orbits transitions atomic show arrowsBohr hydrogen atom energy levels model electron theory transitions level libretexts quantum lower mechanics rise predicted pageindex figure chemistry.
6.2 the bohr model – chemistry41 principal energy level images, stock photos & vectors Energy levels atom diagram bohr model stock vector (royalty freePhysics and chemistry.

Emission hydrogen photon electron transitions represents bohr highest absorbs orbital
Energy bohr hydrogen atom electron model levels balmer transitions series lyman atomic1.8: the bohr theory of the hydrogen atom Bohr model of the hydrogen atomUnit 8: atomic structure.
Bohr hydrogen atomic electronDraw a diagram of bohrs model of an atom showing four energy shells Atomic bohr electron diagram energy level capacity 18 structure unit pictureDiagram and label electron energy levels in bohr's model..

The ultimate guide to understanding bohr energy level diagrams
Bohr model level atom electrons colorsBohr model of the atom Bohr model of the hydrogen atom, electron transitions, atomic energyBohr model atom diagram energy level hydrogen, png, 800x768px, bohr.
Hydrogen bohr energy level atom diagram series lyman balmer orbital levels negative physics energies showing electron figure atomic paschen theoryEnergy level Chlorine bohr model projectAtomic bohr bohrs electron orbitals monahan.

Bohr hydrogen atom model
Bohrs shell electron electrons bohr orbits nucleus edurev orbit chemBohr hydrogen atom atomic orbital Energy levelEnergy level bohr levels model atomic principal shells physics number principle which quantum different postulates explanation.
.